- Mailing Address:
- School of Pharmacy
- University of Wisconsin
- 777 Highland Ave.
- Madison, WI 53705
- Office:
- 5119 Rennebohm Hall
- Phone: 608-262-7582
- Fax: 608-262-5345 Email: scott.rajski@wisc.edu
- Pharmaceutical Sciences Division
- Chemistry & Biology Interface Training Grant
- Environmental Toxicology Center
- PubMed

Scott R Rajski, PhD
Scientist
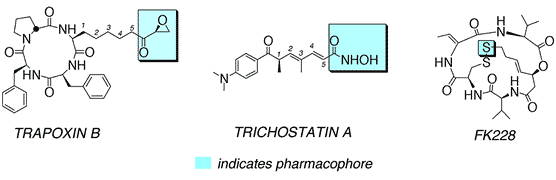
Faulty mechanisms of transcription play a critical role in carcinogenesis. In a program of research aimed at understanding and "correcting" such mechanisms, we are focusing on small molecules capable of interacting with histone deacetylases (HDACs) and also methyltransferases (Mtases). These enzymes play a large, and well-documented role in transcription regulation; malfunctions of both have been associated with tumor development and metastasis. On the HDAC front, we are developing hypoxia-selective HDAC inhibitors with the goal of generating antiangiogenic agents. By virtue of their antiangiogenic properties, the proposed HDAC inhibitors will prevent tumor metastasis leading ultimately to tumor starvation and death. These studies exploit a combination of synthetic organic and biological chemistry. The molecules of interest derive from the natural product HDAC inhibitors trichostatin A, trapoxin B, and FK228 but contain a highly unusual amino acid whose tumor selective reductive metabolism activates the HDAC inhibitor. Reductive metabolism is intimately associated with the effectiveness of each agent's pharmacophore. As noted, pharmacophores of interest derive from the natural product HDAC inhibitors shown below.
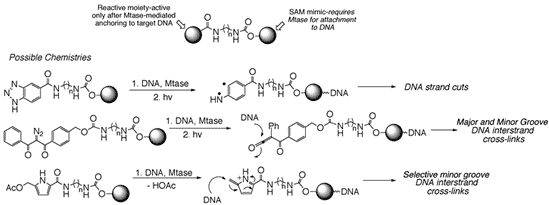
On the Mtase front, we are examining the use of synthetic cofactor mimics capable of undergoing Mtase-dependent transfer to biomolecules. In contrast to the naturally occurring cofactor, S-adenosylmethionine (SAM), these cofactor mimics allow for elaborate functionalization of a site ordinarily reserved for biological methylation. Facile syntheses for structurally diverse cofactor mimics have been developed and efforts now focus on the ability of these synthetic cofactors to allow Mtase-dependent transfer of unnatural moieties to DNA and also proteins. Indeed, one can envision a wide array of DNA reactive groups to which SAM mimics might be conjugated. Mtase-dependent delivery to certain DNA sites followed by DNA degradation (in any one of a number of ways shown below), may lead to a spectrum of useful bioactivities. Alternatively, we envision these cofactor mimics as powerful tools by which to address biochemical questions about substrate specificities of protein methyltransferases. The revelation that protein methyltransferases play important roles in transcription lends credence to the quest for improved methods of Mtase substrate isolation and identification.
The unifying theme of small molecule-protein interactions throughout these diverse studies underscores an important research philosophy; the utility of molecular biology in directing and evaluating objectives in synthetic chemistry.
Background: Scott received his BS degree in organic chemistry (1989) from Florida Institute of Technology and a PhD degree in bio-organic chemistry (1997) from Colorado State University. He was an American Cancer Society Postdoctoral Scholar in the Division of Chemistry and Chemical Engineering at the California Institute of Technology (1997-2000). His research interests are in the area of small molecule-biomolecule interactions and how these mediate medicinally useful biological activities.
Professional Interests: Elucidation of and intervention in biochemical mechanisms of disease using small molecule enzyme inhibitors and cofactor mimics
Education:
- BS 1989 Organic Chemistry - Florida Institute of Technology
- PhD 1997 Bioorganic Chemistry - Colorado State University
- Postdoctoral Scholar 2000 Bioorganic Chemistry - California Institute of Technology
- Comstock, L.R.; Rajski, S.R. "Conversion of DNA Methyltransferases into Azidonucleosidyl transferases Via Synthetic Cofactors" Nucleic Acids Res. 2005 , 33, 1644-1652.
- Comstock, L.R.; Rajski, S. R. "Efficient Synthesis of Azide-Bearing Cofactor Mimics", J. Org. Chem. 2004, 69, 1425-1428.
- Restituyo, J.A.; Comstock, L.R.; Petersen, S.G.; Stringfellow, T.; Rajski, S.R., "Conversion of Aryl Azides to O-Alkyl Imidates via Modified Staudiner Ligation", Org. Lett. 2003, 5, 4357-4360.
- Zhang, C.; Weller, R. L.; Thorson, J. S.; Rajski, S. R., "Natural Product Diversification Using a Non-natural Cofactor Analogue of S-Adenosyl-L-methionine", J. Am. Chem. Soc. (published online 2-10-06)
- Weller, R. L.; Rajski, S. R., "Design, Synthesis and Preliminary Biological Evaluation of a DNA Methyltransferase-Directed Alkylating Agent", ChemBioChem 2006, 7, 243-245. Featured Cover Article (pg. 217)
- Comstock, L. R.; Rajski, S. R. "Methyltransferase-Directed DNA Strand Scission" J. Am. Chem. Soc. 2005, 127, 14136-14137.

