- Mailing Address:
- School of Pharmacy
- University of Wisconsin
- 777 Highland Ave.
- Madison, WI 53705-2222
- Pharmaceutical Sciences Division
- Curriculum Vitae
Email: jeanette.roberts@wisc.edu

Jeanette C Roberts, PhD, MPH
Emeritus
No longer accepting graduate students or postdoctoral fellows.
Medicinal/Pharmaceutical Chemistry. Biological aspects of drug/isotope/toxin targeting using prodrugs; monoclonal antibodies and/or liposomes; thiols and selenols as chemoprotective and chemopreventive agents.
Project 1:
- A major emphasis in my laboratory is the design, synthesis, and preliminary evaluation of prodrugs of L-cysteine. We construct the prodrugs by the chemical condensation of the amino acid with a variety of carbonyl donors to produce thiazolidine rings with various structural features. 2-Oxothiazolidine derivatives require enzymatic biotransformation to release the cysteine, while 2-alkyl analogs spontaneously undergo ring opening and hydrolysis to liberate the desired agent. Once freed from the prodrug, the L-cysteine is available for direct effects, such as free radical scavenging via the thiol functionality, or indirect effects, such as providing the required precursor for glutathione biosynthesis. Prodrug forms are necessary because the free amino acid itself possesses unwanted side effects at protective doses.
- The overarching goal is to protect the body against a variety of toxic agents or situations. We've used the hepatotoxin acetaminophen for several years as a model toxin that requires glutathione for detoxication. In a related area, a new HPLC method was recently completed that allows us to quantitate several thiols of biological interest simultaneously.
- There is growing evidence that effects on the transcription of important genes is an underlying mechanism of action of redox active agents such as cysteine. The upregulation of Phase II drug metabolizing genes (many of which use glutathione as a cofactor) looks like a very promising way to go. We're also particularly interested in exploring effects at the transcriptional level of the enzyme gamma-glutamylcysteine synthetase, the rate-limiting step in glutathione biosynthesis. Our hypothesis is that there must be a coordinate upregulation of glutathione-producing and glutathione-utilizing systems for protection to be optimized.
- Because glutathione is important in so many diseases and toxicities, this approach should be useful against all sorts of things, including solvent exposures, radiation, the side effects associated with many anticancer therapies, environmental pollutants, AIDS, cancer, cataracts, etc.
Project 2:
- In related work, we are attempting to explore structural modifications to the cysteine prodrugs to optimize their ability to enter the cell and provide the amino acid. As a first step, thiazolidine prodrugs are being synthesized in which the free carboxylate group is being replaced by ester or amide functionalities. In this way, the lipophilicity of the compound is increased, hopefully leading to increased residence time in the body, increased cell penetration, and increased protective activity.
Project 3:
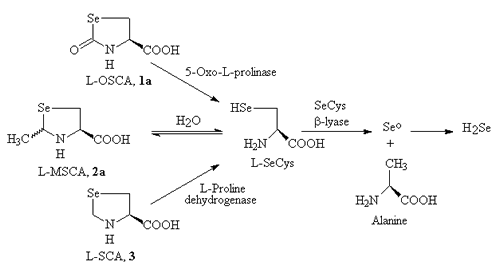
- Our newest project grew out of the thiazolidine prodrug of cysteine work. In this case, however, we are starting with selenocysteine and hence producing Selenazolidine ring forms. Selenium is enjoying growing prominence as a cancer chemopreventive agent and is also of great interest in the AIDS arena. Unfortunately, it gets toxic at levels not terribly far above those required for nutritional uses, and rather high amounts appear to be required, at least for cancer prevention. Therefore, supplementing the body with selenium is a rather tricky business.
- Our hypothesis is that selenocysteine represents the biochemically superior form in which to supply selenium, primarily due to how various selenium-containing compounds are metabolized by the body. [See Figure 1 Below] Unfortunately, selenocysteine is difficult to handle chemically, which has probably deterred its extensive study and use. Applying our prodrug approach [See Figure 2 Below], however, should produce a chemically superior form, greatly facilitating the use of selenium as a cancer chemopreventive or AIDS therapeutic agent. Synthesis of a handful of prototype Selenazolidines is in progress.
Figure 1
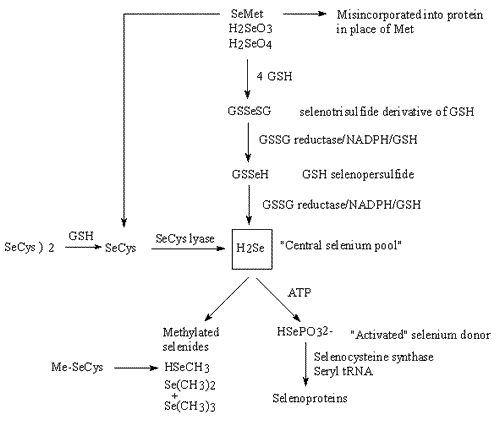
Figure 2
Education:
- BS 1979 - Albright College
- PhD 1986 Medicinal Chemistry - University of Minnesota
- MPH 2001 - University of Utah

