- Mailing Address:
- School of Pharmacy
- University of Wisconsin
- 777 Highland Ave.
- Madison, WI 53705
- Pharmaceutical Sciences Division
- Full list of publications
- Pub Med
Email: WSMellon@wisc.edu

William S Mellon, PhD
Emeritus
No longer accepting graduate students or postdocs
An intriguing question that has perplexed biologists is how hormone-receptor interactions transmit information to target cells to regulate cellular homeostasis. A common mechanism for steroid-like molecules is to bind its cognate receptors which interact with specific nucleotide sequences in the genes that the hormone regulates. The binding of the hormone-receptor complexes to these specific sites activates (and may also suppress) transcription of these genes. Another feature of gene expression that hormone/receptor complexes control is posttranscriptional regulation. While there exists a paucity of information regarding the mechanism whereby this occurs, available information suggests that posttranscriptional modification of mRNA makes a significant contribution to overall gene expression.
Research in my laboratory is directed towards characterizing the receptor dynamics in target cells for 1,25-dihydroxyvitamin D3, the hormonally active form of vitamin D3. The focus of these investigations has been integrated within a broader context of elucidating the control of cellular differentiation of hematopoietic, immune, and bone cells induced by 1,25-dihydroxyvitamin D3. Several clonal cell lines are employed in which we can characterize receptors, quantitate phenotypic and pleotropic changes, ascertain signal transduction pathways, and monitor alterations in gene expression at the transcriptional and posttranscriptional levels. Of late, we have begun to dissect nuclear hormone-receptor control of posttranscriptional regulation of mRNA stability which appears to involve protein kinase C signaling events.
Students in my laboratory can choose several experimental strategies to answer questions relevant to receptor control of cellular events. Biochemical techniques are used to determine structure and examine physical-chemical parameters of receptors. The identification of nucleotide sequences within regulated genes are carried out by utilizing chimeric gene constructs, transfections and various other molecular biological techniques. In addition, we have begun to employ light fluorescence and confocal microscopy to enable delineation of cellular events. The mixture of biochemical/molecular/morphological approaches enable us to provide additional insight into hormonal regulation of gene expression with regard to cellular differentiation.
A working hypothesis model on the mechanism of 1,25(OH)2D3-induced osteocalcin mRNA stabilization. The 5'-UTR of osteocalcin mRNA contains a stem loop structure that spans the entire 5'-UTR and the first 30 nucleotides of the first exon. the stem-loop structure forms a recognition site for specific factors that under normal physiological conditions bind to osteocalcin mRNA and result directly or indirectly in its degradation. 1,25(OH)2D3 results in phosphorylation and therefore inactivation of these cytoplasmic factors which in turn result in stabilization of osteocalcin mRNA.
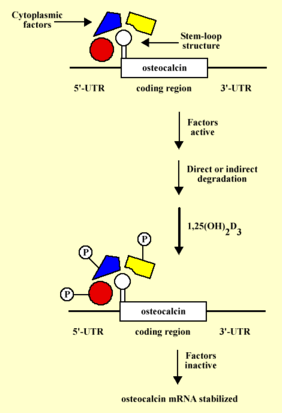
A working hypothesis model on the mechanism of 1,25(OH)2D3-induced osteocalcin mRNA stabilization. The 5'-UTR of osteocalcin mRNA contains a stem loop structure that spans the entire 5'-UTR and the first 30 nucleotides of the first exon. the stem-loop structure forms a recognition site for specific factors that under normal physiological conditions bind to osteocalcin mRNA and result directly or indirectly in its degradation. 1,25(OH)2D3 results in phosphorylation and therefore inactivation of these cytoplasmic factors which in turn result in stabilization of osteocalcin mRNA.
Background: William received his BS degree (1969) in pharmacy from Northeastern University and his PhD degree (1976) in pharmacology from The Ohio State University. He was an NIH Postdoctoral Fellow at the Department of Biochemistry, U.W.-Madison before joining the School of Pharmacy faculty in 1979. His research interests include the biochemical pharmacology of hormone action, with particular emphasis on receptor dynamics and their transcriptional and posttranscriptional regulation of target genes to understand the molecular mechanism of hormone action. A correlative area of investigation involves the elucidation of cellular differentiation of hematopoietic and immune cells induced by sterol hormones and peptides. He is a member of the American Society of Biochemistry and Molecular Biology and the Endocrine Society.
Professional Interests: Biochemical pharmacology of hormone action, with particular emphasis on receptor dynamics and their transcriptional and posttranscriptional regulation of target genes to understand the molecular mechanism of hormone action
Education:
- BS 1969 Pharmacy - Northeastern University
- PhD 1976 Pharmacology - The Ohio State University
- M.A. Rivera-Bermúdez and W.S. Mellon, "1,25-Dihydroxyvitamin D3 [1,25(OH)2D3] translocates PKCa but not PKCb1, PKCe or PKCz to the nuclei in ROS 17/2.8 cells," Endocrinology, abstract #P1-601 (1998).
- R. Mosavin and W.S. Mellon, "Posttranscriptional regulation of osteocalcin mRNA in clonal osteoblast cells by 1,25-dihydroxyvitamin D3," Arch. Biochem. and Biophys., 332, 142-152 (1996).
- K. Mangasarian and W.S. Mellon, "1,25-Dihydroxyvitamin D3 destabilizes c-myc mRNA in HL-60 leukemic cells," Biochem. Biophys. Acta, 1172, 55-63 (1993).
- M. Moss, E.R. Palmer, P. Kuzmic, B.E. Dunlap, W. Henzel, J. Kofron, C. Royer, W.S. Mellon and D.H. Rich, "Identification of actin and HSP70 as cyclosporin A binding proteins by photoaffinity labelling and fluorescent displacement assays," J. Biol. Chem., 267, 22054-22059 (1992).